2,4,6-Trinitrophenol
As the name suggests, 2,4,6-trinitrophenol (TNP) is a tripply nitrated phenol molecule. This high level of nitration results in a compound which is both highly flammable and, under certain circumstances, explosive. The TNP molecule is capable of donating a H+ ion to a base and is therefore considered an acid.TNP was first synthesized (as a phenol derivative) during the early 1800's and it wasn't long before it was being used in munitions. By the time World War I had started TNP was being used extensively in munitions (artillery, bombs, torpedoes etc) by most sides concerned.
When TNP, an acid comes into contact with a metal a reaction can take place which results in a salt. This process is true of any acid and metal, but special consideration is needed when dealing with TNP, and more specifically, the salts of TNP as some of them are extremely shock sensistive explosives and are therefore something that should be avoided at all costs. It was the undesirable formation of these TNP salts that led to TNP being replaced by the more stable TNT (2,4,6-trinitrotoluene). Infact, by the time World War II had started most countries had already scraped TNP in favour of TNT.
Mechanism
I think the reactions that take place during the synthesis of TNP are especially interesting as they provide a certain amount of complexity. The following is my attempt at showing the mechanism of the synthesis and therefore could contain some errors. If you believe the mechanism is different then feel free to send me an email.The mechanism starts with 2-acetoxybenzoic acid being broken down into salicylic acid and acetic acid.
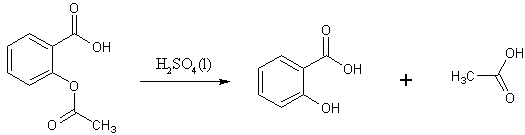
The salicylic acid then undergoes decarboxylation to form phenol and CO2.
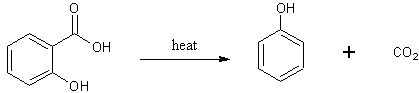
The phenol is then sulphonated to form both o-phenolsulphonic and p-phenolsulphonic.
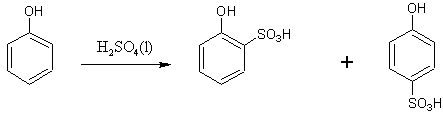
Both the o-phenolsulphonic and p-phenolsulphonic molecules go through nitration to form 2,4,6-trinitrophenol, but they each take a slightly different path.
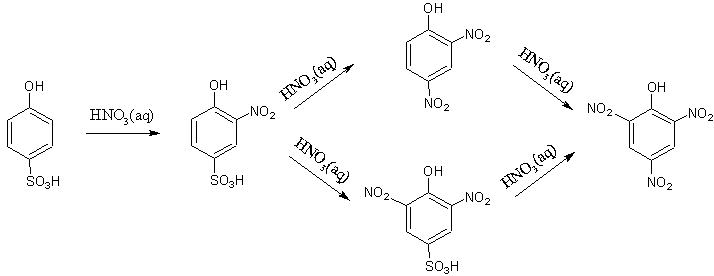
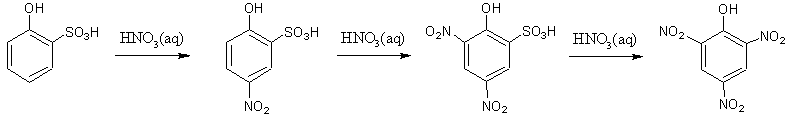
Synthesis
During the synthesis of 2,4,6-trinitrophenol the solution goes through some interesting colour changes, which are demonstrated in the following images. The descriptions associated with the images are delibrately vague so as not to act as a how-to guide and therefore do not included chemical names or quantities.The white object in the pictures is the top of my hot plate which also includes a magnetic stirrer. The magnetic stirrer was turned on through out the synthesis. In some of the pictures the hot plate heater is actually turned off and the solution is running at a reduced temperature.
The synthesis begins with the formation of phenol which goes on to become phenolsulphonic acid.
 |
 |
The second stage of the synthesis is the nitration of the SO3H groups followed by cooling.
 |
 |
 |
 |
The solution then goes through a process to encourage crystal growth.
 |
 |
 |
 |
The 2,4,6-trinitrophenol crystals are then vacuum filtered and washed several times. The final 2,4,6-trinitrophenol is kept under an excess of distilled water.
 |
Safety
2,4,6-trinitrophenol is a very dangerous compound and needs very special consideration during synthesis, use, and disposal. I strongly discourage anyone from using this acid unless it's absolutely necessary. For detailed and accurate safety precautions the relevant MSDS should be referred to. Nothing on this website should be used as a guide to its synthesis. Professional sources should be referred to before attempting anything similar.If 2,4,6-trinitrophenol is allowed to dry out it will become an explosive compound. For this reason it should always be stored under liberal amounts of distilled water. The salts of 2,4,6-trinitrophenol are extremely shock sensitive high explosives and therefore contact with metals should be avoided at all costs.
| Home |